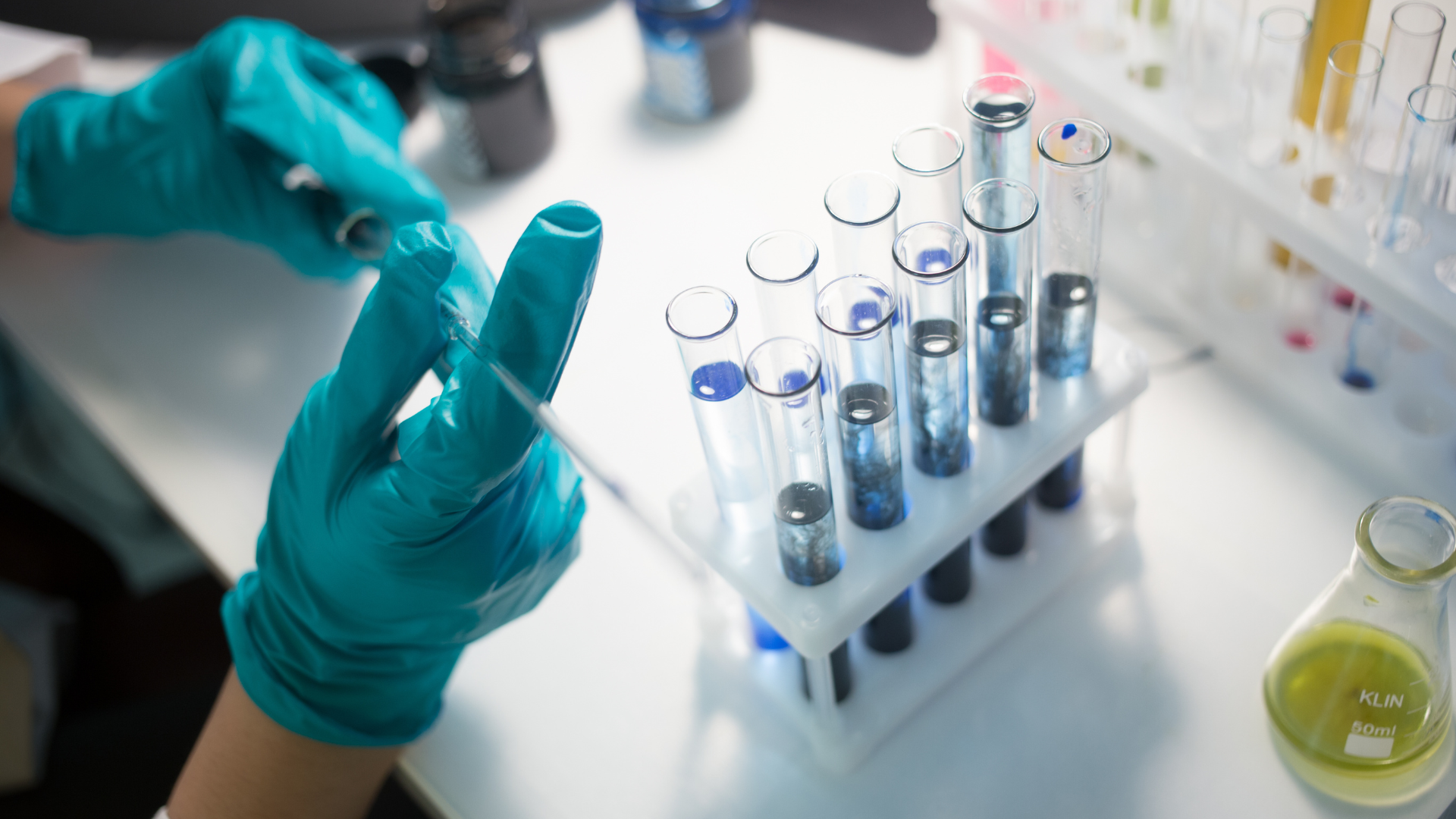
Impurities Analysis and Management
Impurities are unwanted products that remain with active pharmaceutical ingredients (APIs) and medicines. The presence of these impurities even in trace amounts may influence the efficacy and safety of pharmaceutical products. Identification and quantification of these impurities (Impurity Profile) are now becoming an important critical product quality parameter and are getting critical review from regulatory authorities. Because of this, the limit and threshold values for impurities are specified by pharmacopeias and ICH guidelines. Analysis of impurities according to this set of specifications is mandatory for the pharmaceutical product release for human consumption. Impurity Profile study is also incredibly important for market approval and for finished dosage forms from regulatory authorities.
The identification and quantification of impurity in pharmaceutical products are carried out by using certified impurity standards of known purity. It is recommended to use an impurity standard of >90% purity when used for peak identification in the chromatogram or use in the system suitability test for resolution, and a minimum of 95% purity when used to estimate the content of a specified impurity.
Sambi Pharmaceuticals offers a wide range of high purity and impurity standards for pharmaceutical industries on the mg to kg scale at competitive prices. Sambi Pharmaceuticals has a technical capability to synthesize, isolate, and purify the impurity standards to their purest form. Sambi Pharmaceuticals can also offer the impurity standards from mg to gm scale.
Lab Services:
-
-
-
- Synthesizing NCEs
- Library Synthesis for LI N LO Projects
- Our Business Model Includes FTE n FFS Hybrid Models, Long term Multiyear contract and continuous material supply support.
-
-
Our Expertise in handling:
-
-
-
- Heterocycles
- Chiral Chemistry – Chiron & Asymmetric synthesis
- Unnatural amino acids
- Small peptides: Solution phase up to hexamers
- Carbohydrates & Aza sugars
- Nucleoside building blocks
- PROTAC Chemistry
- ADC Linkers
- PEG- Lipids
-
-
Analytical chemistry:
-
-
- Data analysis & interpretation
- Method development & validation
- Reporting the results & documentation
-
Quality Assurance and Quality Control:
-
-
- At Sambi Pharma, utmost importance is given to Quality than business profits. With our quality culture across the company, we have built a remarkable reputation with clients.
- Every member of our team is equally committed to delivering zero-defect products on time, and as promised to our customers.
- The Principles of the Quality Management System adopted by the company are as per ISO 9001:2015 guidelines.
-
Process R&D & Manufacturing:
-
-
-
- Chemical Synthesis – Route proposal, Selection and Synthesis
- Process Development – Reaction optimization, Scale up studies, Product purification, Safety studies, Quality control, Process validation and Technology transfer.
- Scale up & Manufacturing: Synthesis of Advanced API intermediates and KSM (Key Starting Material) from KG-MT Scale.
- Loan licensed GMP/Non-GMP plants to offer commercial manufacturing activities.
-
-
Our In- House Pilot Plant Includes:
-
-
-
- 100 L 200 L 500 L glass reactor with Heating/cooling system: –70°C to +150°C
- 20 L high vacuum distillation unit
- Pressure Nutsche filter, centrifuge
- Autoclave ranging from 100ML – 25 L
- Rotavapors ranging from 2L – 20 L
- Vacuum Tray dryer
-
-
Our Infrastructure
Why work with Sambi Pharma
Sambi Pharmaceuticals offers custom synthesis of mg to gm level of impurity compounds on requests. In case of non-standard impurity, the standard additional study for structure elucidation, characterization, and analytical method validation are also undertaken using elemental analysis and state-of-the-art equipment like; LC-MS, HPLC, 1 H and 13 C NMR, FT-IR.
Nam at augue non risus interdum suscipit at a sapien. Nunc lobortis egestas massa sit amet bibendum.
Contact
4953 Vine Street
San Diego, CA 92465
815.555.5555
info@sambipharma.com