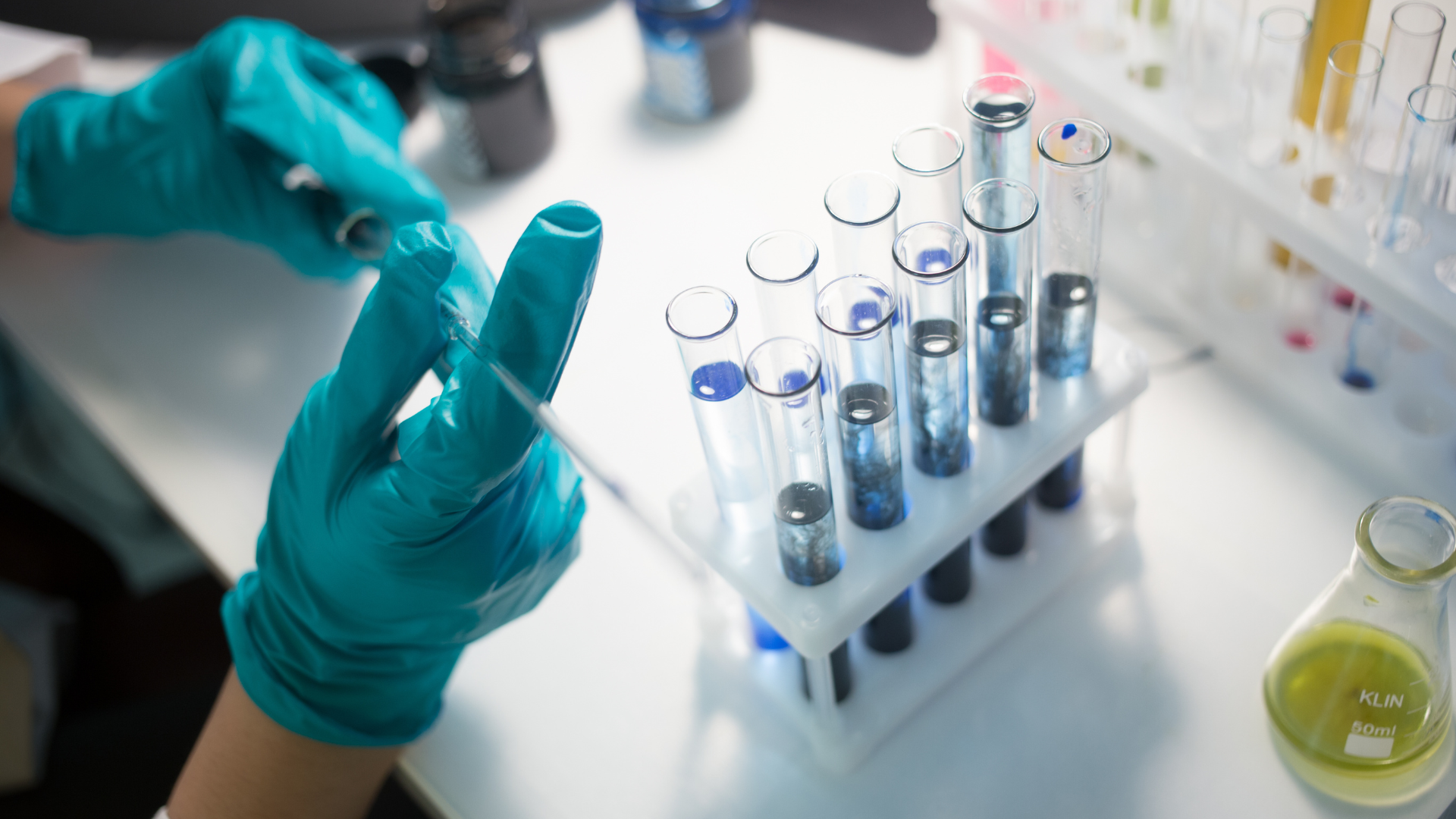
Discovery (CRO) Services
Sambi Pharma offers a broad range of contract research services which are forward integrated with our API services. All carried out at our state-of-the-art research center. Our team is composed of highly motivated scientists who trained at prestigious universities across the world. Our experienced team can perform a wide range of chemistry services such as:
- Asymmetric synthesis
- Organometallic chemistry
- Metal mediated transformations
- Nucleotide chemistry
- Carbohydrates
- Macro cyclic compounds
- Fluorine and boron chemistry
- Visible light chemistry
- Electrochemical reactions
- High pressure reactions
- Cryogenic reactions
Lab Services:
-
-
-
- Synthesizing NCEs
- Library Synthesis for LI N LO Projects
- Our Business Model Includes FTE n FFS Hybrid Models, Long term Multiyear contract and continuous material supply support.
-
-
Our Expertise in handling:
-
-
-
- Heterocycles
- Chiral Chemistry – Chiron & Asymmetric synthesis
- Unnatural amino acids
- Small peptides: Solution phase up to hexamers
- Carbohydrates & Aza sugars
- Nucleoside building blocks
- PROTAC Chemistry
- ADC Linkers
- PEG- Lipids
-
-
Analytical chemistry:
-
-
- Data analysis & interpretation
- Method development & validation
- Reporting the results & documentation
-
Quality Assurance and Quality Control:
-
-
- At Sambi Pharma, utmost importance is given to Quality than business profits. With our quality culture across the company, we have built a remarkable reputation with clients.
- Every member of our team is equally committed to delivering zero-defect products on time, and as promised to our customers.
- The Principles of the Quality Management System adopted by the company are as per ISO 9001:2015 guidelines.
-
Process R&D & Manufacturing:
-
-
-
- Chemical Synthesis – Route proposal, Selection and Synthesis
- Process Development – Reaction optimization, Scale up studies, Product purification, Safety studies, Quality control, Process validation and Technology transfer.
- Scale up & Manufacturing: Synthesis of Advanced API intermediates and KSM (Key Starting Material) from KG-MT Scale.
- Loan licensed GMP/Non-GMP plants to offer commercial manufacturing activities.
-
-
Our In- House Pilot Plant Includes:
-
-
-
- 100 L 200 L 500 L glass reactor with Heating/cooling system: –70°C to +150°C
- 20 L high vacuum distillation unit
- Pressure Nutsche filter, centrifuge
- Autoclave ranging from 100ML – 25 L
- Rotavapors ranging from 2L – 20 L
- Vacuum Tray dryer
-
-
Our Lab Images
Possibilities are endless, due to our State-of-the-art infrastructure:
-
-
-
- State-of-the-art research labs and analytical facility
- Separate work station adjacent to the lab for record writing
- e-LNB / p-LNB documentation protocols followed as per client requirement
- non-GMP-Kilo-Lab
-
-
Sambi Pharma Advantage
-
-
-
- Early phase process development.
- Late phase process development.
- Commercial scale process development.
- API process development.
- Life cycle management.
-
-
Nam at augue non risus interdum suscipit at a sapien. Nunc lobortis egestas massa sit amet bibendum.
Contact
4953 Vine Street
San Diego, CA 92465
815.555.5555
info@sambipharma.com